
About the Author
Mohammed Jobran
Regulatory consultant with +20 years of experience working for the SFDA, multinational companies, and as a consultant in PharmaKnowl.
Navigating the SFDA medical device registration in Saudi Arabia, particularly the Medical Device Marketing Authorisation (MDMA) process, is essential for manufacturers seeking entry into the rapidly expanding Saudi market.
The MDMA requirements have evolved to cope with the advancement of international standards, such as the evolution from EU Medical Device Directives (MDD) to Medical Device Regulations (MDR). The continuous development of medical device regulations in Saudi Arabia has increased the complexity of this process.
For example, even low-risk medical devices (supplies and consumables) became in need of an authorised representative (AR) and an MDMA approval.
We at PharmaKnowl offer expert support for the registration process. Whether you’re dealing with new devices or modifying existing products, our team provides the strategic insight and technical expertise needed to achieve timely and successful market entry in Saudi Arabia.
This article clarifies the SFDA medical device registration requirements and defines their classification differences.
For other types of product registration, refer to the SFDA registration post.
Table of contents
Registration Applications
The SFDA regulates medical device products in Saudi Arabia to ensure their safety, efficacy, and quality. The manufacturers must acquire MDMA approval before marketing their products. Post-approval, they are responsible for safety reporting and other life cycle management operations, such as update, renewal, and Unique Device Identification (UDI) submission.
Here is a summary of the registration procedures for medical devices:
MDNR (Low Risk) – Cancelled
Companies used to register Non-sterile, non-measuring, Low-Risk Medical Devices through the Medical Device National Registry (MDNR), also known as “Medical Devices Listing,” which is exempt from MDMA and AR. However, in September 2022, the SFDA cancelled the MDNR procedure. Therefore, all devices must have a technical file for MDMA with an appointed AR in Saudi Arabia.
MDMA 1 (GHTF) – Cancelled
In this application route, the SFDA considered approvals from the GHTF member countries, such as the European Union, the United States, Canada, Australia, and Japan. It was an easy route to follow without deep technical inquiries. However, by the end of 2021, the SFDA had cancelled this GHTF route.
MDMA 2 (TFA)
Since January 2022, all medical devices and in vitro diagnostics (IVDs) must receive an SFDA authorisation through the Technical File Application (TFA) registration route (MDMA2). This route resulted from the authority’s continuous regulation updates influenced by the EU MDR and IVDR. It requires presenting the technical documentation in a clear, searchable, and organised manner.
Therefore, the registration requirements became more stringent. Many conditions must be met, and studies must be provided, such as clinical evaluation reports (CER), biocompatibility test reports, and the need to conduct a post-market clinical follow-up (PMCF) study in Saudi Arabia.
Although the TFA requirements are similar to those of the EU (CE mark), the SFDA does not require a CE certificate. However, if the product file doesn’t carry a CE mark, it will undergo a rigorous assessment.
Registration Requirements
The following summarises the requirements, which vary according to the product type and class. Here are the high-level sections of the registration file:
- Table of contents
- Device description
- Intended use/purpose
- Device history
- Device Classification.
- Device label
- Device Models, Accessories, and Variants
- Instructions for use
- Design Information
- Manufacturing Information
- Essential Principles (EP List) of Safety & Performance (Essential Required checklist)
- Benefit-risk analysis
- Risk Management File (Plan & Report)
- Pre-clinical testing
- Testing reports
- Biocompatibility Test Reports.
- Clinical investigation plan & report
- Clinical Evaluation Report (CER)
- Post Market Clinical Follow Up (PMCF)
- Post Market Surveillance (PMS), Plan & Report
- Periodic Safety Update Report (PSUR) for medical devices.
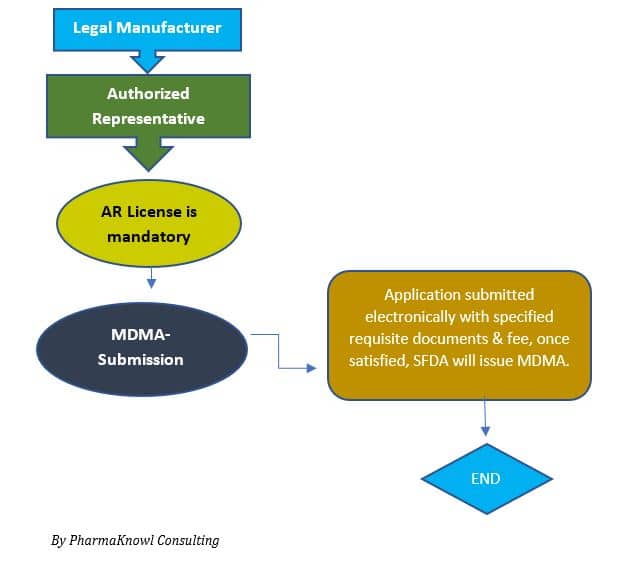
Registration Process
Time needed: 90 days
We can summarise the medical device registration (MDMA) approval process in the following few steps:
- Appointing an Authorised Representative (AR)
Manufacturers must appoint an AR to represent their company in Saudi Arabia.
- Gap Analysis
Companies must establish a thorough gap analysis of the registration file vs. all applicable requirements. Identifying the gaps is essential to determining the relevant studies and tests, such as Stability, CER, Biocompatibility, risk management, and PMCF.
- MDMA Submission
The applicant submits the technical file to the SFDA.
- Validation
SFDA ensures good submission practice without assessing its content.
- Fees Payment
The applicant will receive an SFDA invoice before the assessment.
- Application Assessment
The assessors will review the file and send back their inquiries in multiple waves.
- MDMA Approval
The legal manufacturers receive an MDMA certificate that permits them to market the product in Saudi.
Classification
Applicants must classify their products according to the SFDA medical device classification rules within the MDMA application. These rules correspond to the EU regulation (CE certificate) since they are the unofficial SFDA reference regulations, but do not necessarily match them.
Medical Device
SFDA medical device classification is either Class A, B, C, or D. It is based on the risk class. The class is necessary to determine the registration procedure and its requirements, as discussed in the table below:
| SFDA Classification | Risk Class | MDR Classification Rule |
| Class A | Low | I |
| Class A – Sterile | Low-medium | Is |
| Class A – Measuring function | Low-medium | Im |
| Class A – Reusable surgical instruments | Low-medium | Ir |
| Class B | Low-medium | IIa |
| Class C | Medium-high | IIb |
| Class D | High | III |
Classification is according to the following:
- Intended use.
- Risk level (harm probability and severity to the patients, users, and others).
- Invasiveness to the human body.
- Use duration.
Identical devices could be classified differently according to the targeted part of the body. Therefore, the intended use is crucial in determining the correct classification. The intended use reference is reflected in the following:
- Instructions for Use (IFU)
- Label
- Promotional materials
- Technical File
In Vitro Diagnostics (IVDs)
Concerning In Vitro diagnostics, the SFDA is also adopting the European medical device regulation IVDR:
| SFDA IVD Classification | Risk Class | Classification Rule |
| Class A | Low individual risk and low public health risk | A |
| Class B | Moderate individual risk and/or low public health risk | B |
| Class C | High individual risk and/or moderate public health risk | C |
| Class D | High individual risk and high public health risk | D |
The applicant is responsible for determining the class of the IVD by:
- Applying the classification rules for IVD medical devices, and
- Considering:
- Intended use
- Level of risk
Grouping Multiple Devices
Grouping multiple devices in the same application is permissible; the applicant may bundle up to 50 items in one MDMA application. However, products must meet several conditions, such as having the same risk class and intended use.
Timelines
The assessment timelines vary according to many factors, such as risk class, complexity of the product, number of bundled products, and the completeness of the file. Unidentified gaps before the submission will risk delaying the approval. The entire timeline for the registration project includes:
- Gap Analysis Time
This part depends on the number and type of gaps, the speed of manufacturer feedback, and the regulatory staff’s experience. - SFDA Assessment Time
For the official review timelines, refer to our article SFDA timelines.
Fees
The MDMA application fees vary according to the medical device risk Class and the number of products in the application. For more information, refer to our updated SFDA Fees post.
Renewal
MDMA’s default validity is three years. When the license is near expiration, manufacturers can submit an MDMA renewal application up to three months (90 days) before the expiration date. If registered in the obsolete MDMA1 (GHTF) application, the renewal process would be long and require extensive requirements, just like a new registration. In contrast, MDMA2 renewal will be faster.
Update
To make changes to the registered medical device, manufacturers must submit an MDMA update application.
MDMA Certificate
SFDA will issue an MDMA certificate in both Arabic and English containing the following:
- The manufacturer’s information (License Holder)
- Medical device name and medical device group information.
- Validity Period
- Certificate number

Product Categories
Here are examples of medical device types and categories in Saudi Arabia:
- Diagnostic Devices:
- Imaging Equipment: X-ray machines, CT scanners.
- Monitoring Devices: ECG machines and blood pressure monitors.
- In Vitro Diagnostic Devices: Pregnancy tests, blood glucose meters.
- Therapeutic Devices:
- Respiratory Devices: Ventilators, nebulisers.
- Infusion Devices: Insulin pumps and IV therapy equipment.
- Rehabilitation Devices: Wheelchairs, prosthetics.
- Implantable Devices:
- Cardiovascular Devices: Pacemakers, stents.
- Orthopaedic Implants: Hip replacements, spinal rods, joint replacements.
- Surgical Instruments:
- Cutting Instruments: Scalpels, surgical scissors, forceps.
- Staplers and Sutures: Surgical staplers, absorbable sutures.
- Dental Devices:
- Restorative Materials: Dental crowns, fillings.
- Orthodontic Appliances: Braces, retainers.
- Other: dental drills
- Ophthalmic Devices:
- Corrective Lenses: Contact lenses, eyeglasses.
- Surgical Equipment: LASIK machines, intraocular lenses.
- Medical Software (SaMD):
- Diagnostic Software: AI for image analysis.
- Treatment Planning Software: Radiation therapy planning tools.
- Laboratory Equipment:
- AnAnalysersBlood gas ananalysersnd haematology ananalysers.
- StSterilisationquipment: Autoclaves, UV ststerilisers.
- Medical Gases
- Home Healthcare Devices:
- Monitoring Devices: Blood glucose monitors and digital thermometers.
- Mobility Aids: Walkers, electric scooters.
- Therapeutic devices (e.g., dialysis machines)
Medical Device Importing License (MDIL)
Importing medical devices requires a valid MDMA. However, the SFDA may exempt some medical devices from registration and only require them to issue a medical device importing license (MDIL). For example:
- Demonstration or training purposes, medical devices.
- Chemicals (finished product), whether classified as a medical device or used along with medical devices (e.g. gases used to calibrate medical devices, as well as chemicals used to ensure the sterilisation operation of medical devices, manufacturing of prostheses and preservation of tissues or cells). They are excluding the following products (if the chemical is classified as a medical device and the device must have MDMA).
- Radioactive materials
- Medical IVD
- Non-medical IVD
- Chemical precursors.
- The distillation apparatuses for healthcare providers or educational facilities.
- Research and Educational use products.
- Semi-finished medical devices/supplies (and raw and non-raw chemicals) for local manufacturing (manufacturing includes refurbishing, assembling, packaging, and labelling).
Company Registration
The SFDA also regulates medical device companies, enforcing different requirements for local and international companies. Below are the requirements for both types:
Local Saudi Company
The SFDA licenses local medical device companies such as importers, distributors, warehouses, authorised representatives, and manufacturers. The local company license name is the Medical Device Establishment License (MDEL). All previous types of companies must implement a quality management system (QMS) and have an ISO 13485 certificate.
Legal Manufacturer
International legal manufacturers must appoint an authorised representative (AR) in Saudi Arabia; this is the first step in enabling communication with the SFDA. The AR’s primary responsibility is product compliance in pre- and post-marketing activities. The AR will maintain registration status, facilitate shipments, monitor safety, report cases, and represent the company in regulatory or legal matters.
Registration Service
Whether your company is new to or already active in the Saudi market, PharmaKnowl will help you comply with the SFDA and facilitate your business by providing compliance, insights, and supply management to your distributors without commission.
PharmaKnowl is an SFDA-licensed service provider in Saudi Arabia that represents well-known international companies. Contact us to schedule an exploratory meeting or request more information.







