The Saudi Food and Drug Authority (SFDA) requires medical device manufacturers to appoint an authorised representative (AR) in Saudi Arabia before marketing their products. The AR will be the local representative responsible for regulatory activities such as medical device registration and postmarketing surveillance.
Appointing an authorised representative requires submitting an AR application to SFDA to receive an AR license (ARL). It is a fast process, and the manufacturer can change the authorised representative whenever needed without losing the previously approved product licenses (MDMA).
Here are a few important notes:
- Appointing an AR is a 1-2 week process.
- Changing the AR is possible at any time.
- Manufacturers can appoint several ARs (with conditions).
- A valid AR license is necessary before device registration.
- Shipment clearance requires a valid AR license.
This post will discuss the AR role and how to appoint one for your company.
Table of contents
What is an authorised representative?
The authorised representative is the local Saudi company that legally represents the international medical device manufacturer in Saudi Arabia. SFDA requires companies to appoint an AR before registering their product; the AR is not necessarily the distributor.
Independent AR vs Distributor as AR
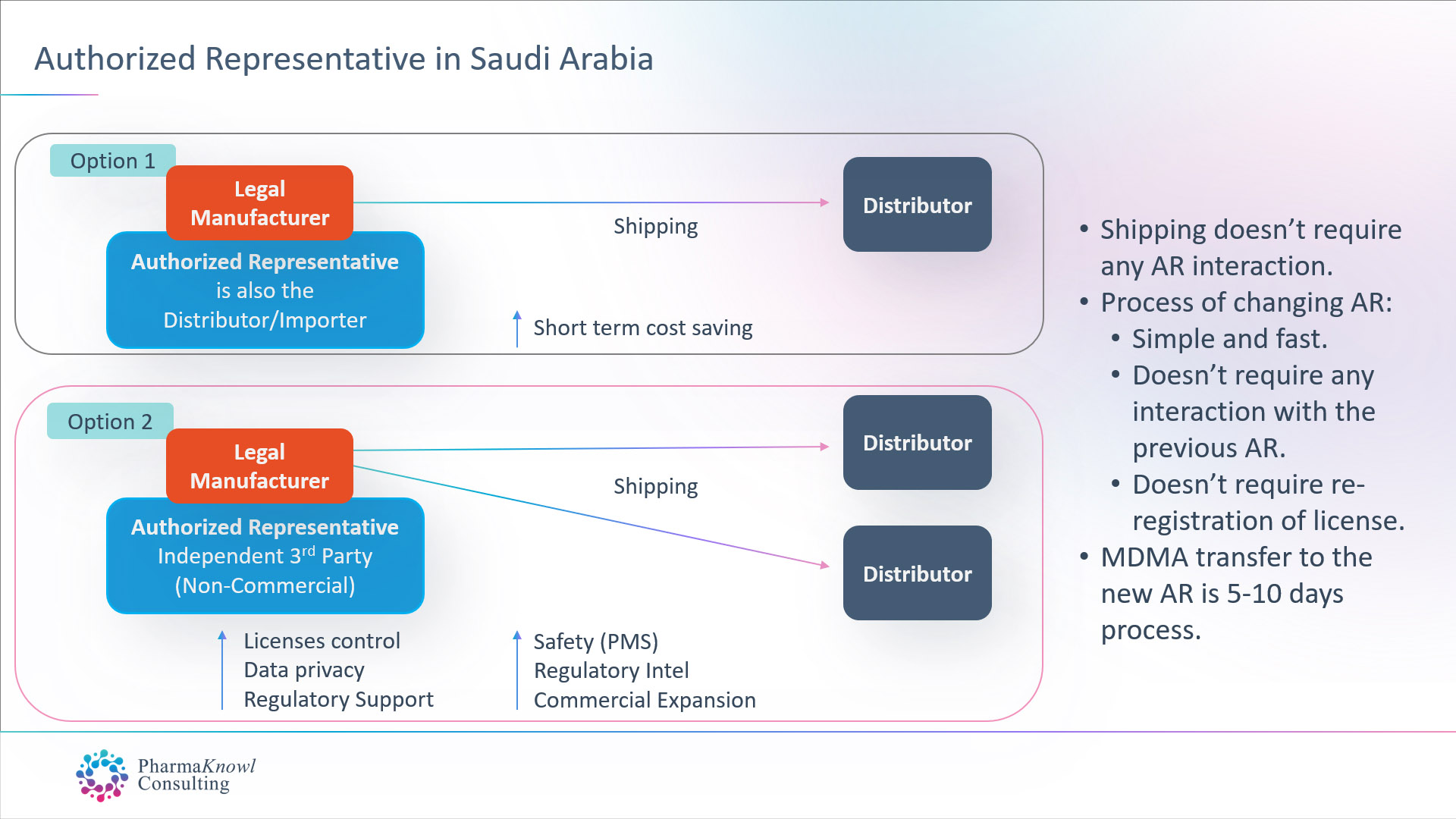
International medical device manufacturers have two options when they want to appoint an AR in Saudi Arabia:
- Appoint their commercial distributor to also act as their AR.
- Appoint an independent AR (non-commercial).
Most legal manufacturers prefer to appoint an independent AR as the best option in Saudi Arabia, similar to the common practice in other regions such as the EU.
Independent AR advantages
When contracting with an independent AR for a fixed annual fee without commission on shipments, manufacturers receive a group of advantages, such as:
- Advanced regulatory and post-marketing support.
- Ability to register multiple brand names for the same product.
- Ability to ship goods to multiple distributors/Importers.
- Stable business operations.
- Control over product licenses
- Data confidentiality.
- Unbiased advice and market insights.
- On-time regulatory intel.
- Advanced communication with the authorities.
- Advanced quality, safety, and maintenance Support
Responsibilities
The AR provides the following to the legal manufacturer:
- Legal, authorised representation at SFDA
- Regulatory Support
- Submit the Unique Device Identification (SFDA UDI)
- Clarification of SFDA regulations
- Market Intel & insights
- License maintenance, such as MDMA Updates and renewals
- Post-marketing Surveillance
- Reporting adverse events and incidents
- Submission of corrective actions
- Conduct post-marketing clinical follow-up (PMCF) in Saudi Arabia
- Facilitate device maintenance
- Support shipment clearance issues
AR Standard Qualities
- Excellent coverage of essential duties
Regulatory compliance, safety monitoring, Communication skills, etc. - Regulatory Intelligence:
Promptly share regulatory updates with the ability to identify impact areas and risk levels. - Good access to SFDA
For support and problem-solving. - Supply chain planning capabilities
E.g., for arrival schedules vs approval dates and import permits - Work within the manufacturer’s internal e-systems
To update regulatory milestones such as registration dates, expirations, renewals, etc. - Support SFDA inspections
- Facilitates shipment paperwork at the port.
- Resolve other in-market issues.

Low-risk classes
In the past, SFDA required AR for high-risk classes only. However, since September 27, 2022, AR has become mandatory on low-risk devices as well, due to the cancellation of the low-risk registration procedure (MDNR).
License Requirements
Here, we list the SFDA requirements for a local company to act as an authorised representative:
- A Saudi company with a Commercial Record (CR) that reflects an acceptable economic activity as per the SFDA for medical devices.
- Availability of a Quality Management System (QMS)
- A valid ISO 13485 certificate.
- Pass the SFDA inspection.
- A valid SFDA medical device establishment license (MDEL)
- Dedicated staff for safety and regulatory functions.
- Signed an authorised representative agreement with the legal manufacturer.
AR Agreement
The SFDA mandates a specific AR agreement template that needs to be signed as it is between the legal manufacturers and their Saudi authorised representative.
Here are a few essential related notes:
- Manufacturers can appoint several ARs (with conditions).
- AR Agreement must be apostilled or legalised.
- The minimum AR validity is one year.
- The AR validity matches the agreement duration or is shorter.
Download: Authorised Representative Agreement Template.
Changing Your Authorised Representative
Changing the authorised representative in Saudi Arabia does not require approval from the previous AR. The process takes one week, followed by the transfer of the medical device licenses (MDMA). Therefore, there is no need to re-register the products after transferring the AR.
Fees
The SFDA license fee for an authorised representative certificate is SAR 2,600/year (USD 693.33). For more details about SFDA charges, refer to our article, “SFDA Fees“.
Authorised Representative Provider
PharmaKnowl is an SFDA-licensed authorised representative trusted by famous medical device and MedTech companies worldwide. If you need an AR for your first market entry or to transfer from a current AR in Saudi Arabia, please contact us to receive a proposal. You can also schedule a meeting with us to answer your questions.
Read More
SFDA Registration
About the Author
Regulatory consultant with +20 years of experience working for the SFDA, multinational companies, and as a consultant in PharmaKnowl. LinkedIn
Resources
Services
Events