In the realm of pharmaceutical innovation, breakthrough medicines stand as transformative agents, promising novel solutions to address critical medical needs. The Saudi Food and Drug Authority (SFDA) recognizes the significance of these groundbreaking therapies and has established a special drug registration procedure to facilitate their development and expedite their regulatory approval. Let’s delve into the key aspects of the SFDA’s approach to breakthrough medicines.
Breakthrough medicines represent a paradigm shift in the treatment landscape, offering substantial advantages over existing therapies for serious or life-threatening conditions. These medicines are those that demonstrate a significant improvement in efficacy or safety compared to available treatments, potentially altering the course of patient care.
The SFDA’s commitment to advancing public health is evident in its proactive stance towards breakthrough medicines. By recognizing the unique challenges and opportunities associated with these transformative therapies, the SFDA aims to streamline their development and regulatory approval processes.
To qualify for breakthrough designation, a drug must meet specific criteria outlined by the SFDA as below:
The SFDA encourages open communication and collaboration between drug developers and regulatory authorities. This collaborative approach involves the creation of development plans that outline key milestones, ensuring transparency and alignment between all stakeholders.
The SFDA exercises flexibility in regulatory requirements for breakthrough medicines while maintaining a robust scientific evaluation, as the applicant may request an exemption for one or more of the registration requirements if unavailable (e.g. CPP, leaflet and artwork).
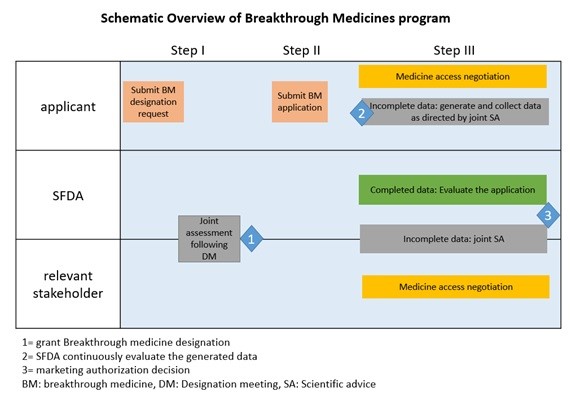
Even after regulatory approval, the SFDA remains actively engaged in post-market monitoring of breakthrough medicines. This ongoing surveillance ensures the continued safety and efficacy of these therapies, addressing any emerging concerns promptly.
The SFDA’s commitment to breakthrough medicines has a profound impact on patient care in Saudi Arabia. By expediting the development and approval of transformative therapies, the SFDA contributes to the availability of cutting-edge treatments, offering new hope to patients facing severe or life-threatening conditions.
In embracing the era of breakthrough medicines, the SFDA positions itself as a facilitator of innovation and a guardian of public health. The guidelines for breakthrough designation not only encourage the development of transformative therapies but also exemplify the SFDA’s dedication to ensuring that these advancements reach patients promptly and responsibly.
As the landscape of medical innovation continues to evolve, the SFDA’s commitment to breakthrough medicines remains steadfast, promising a future where groundbreaking therapies become more accessible, shaping a healthier and more resilient Saudi Arabia.
We support global Pharma and Medical Devices companies in the Saudi market with professional regulatory services. Send us your request to kick off your SFDA project.
Start Here