Trusted by Industry Leaders















Meet Us at Industry Events
Connecting with Our Partners and Regulators Worldwide
Nothing Found
Expertise – Trust – Excellence
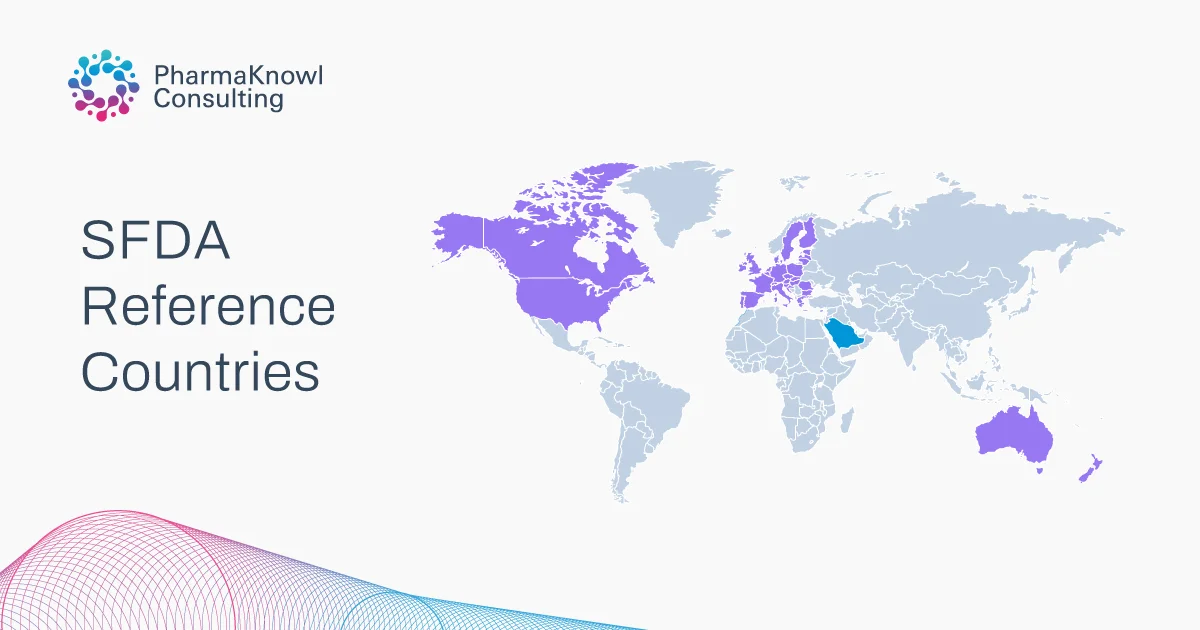
Our Strengths, Your Regulatory Advantage
Combining regulatory expertise, industry versatility, and trusted local presence, we deliver high-impact SFDA regulatory solutions. Our qualified team, cross-sector background, and up-to-date knowledge empower your business to meet compliance requirements with speed and confidence.
Services & Solutions
Explore Our Solutions & Services for Life Science Industries
Resources
Download Expert-Crafted Resources
Nothing Found
Regulatory Insights
Your Trusted Regulatory Intel Source
Nothing Found
Most Recent
Company Updates